The Grignard reagent is one of the most useful organometallic reagents used in organic synthesis. The advantage of a polar C-Mg bond makes it a versatile carbanion source or a nucleophile for the addition reaction. The electropositive nature of Mg attributes to being alkaline earth metal from a second group of the periodic table. And hence polarity of the C-Mg bond depicts the relative negative charge on the carbon. This results in carbon acting as a nucleophile.
So let’s first see how to prepare a Grignard reagent:
In the Grignard reagent, we have two parts, one the alkyl group and the Magnesium halide salt. Upon reaction of Mg metal with an alkyl halide in dry ether gives the respective Grignard reagent.
General reaction:
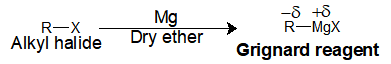
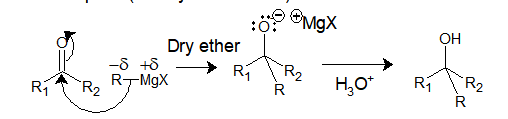
Now let’s see the general mechanism of addition of Grignard reagent with an electrophile (aldehyde or ketone):
Why Dry ether?
Traces amount of water may destroy the Grignard reagent as Grignard reagent act as a strong base.
Addition of Grignard reagent to an electrophile:
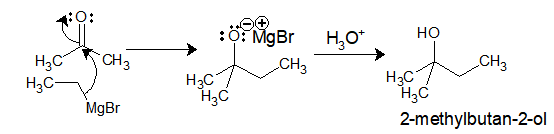
Ethyl-magnesium bromide upon reaction with acetone gives 2-methylbutan-2-ol.

Examples:
I) Addition of Grignard reagent to the Aldehyde/Ketone:
Ethyl-magnesium bromide upon reaction with acetone gives 2-methylbutan-2-ol.
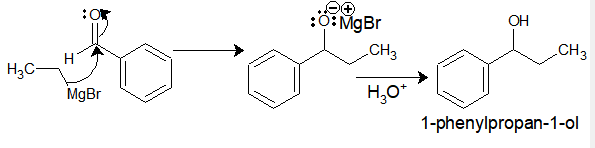
Ethyl-magnesium bromide upon reaction with benzaldehyde gives 1-phenylpropan-1-ol.
II) Addition of Grignard reagent to the Esters:
Ethyl-magnesium bromide upon reaction with Ethylacetate gives 3-methylpentan-3-ol.
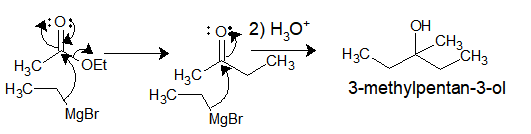
So, Why it gives tert. alcohol instead of a ketone?
So the answer lies in the reactivity of product formed after the first addition. The ethylMgBr attacks as a nucleophile to ester carbonyl to give ethylmethylketone. However, the ketone/aldehyde carbonyl is more electrophilic than ester carbonyl and hence the ketone formed also reacts with another molecule of Grignard reagent followed by acid hydrolysis leading to tert. alcohol.
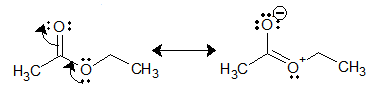
This lower reactivity of ester carbonyl can be attributed to resonance stabilization.
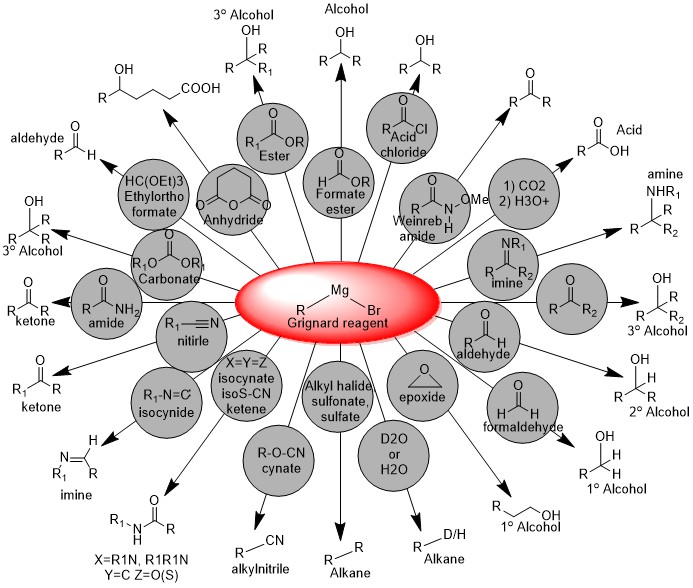
Majority of reactions as a nucleophile are given below:


2 responses to “Blog-03: Grignard reagent and its reactions”