First thing first:
What is chiral carbon?
When carbon is attached to the four different atoms or groups of atoms then the carbon is called a chiral carbon.
Why is it important to know the configuration of a chiral carbon?
Take an example of ibuprofen used as a “pain-killer”, contains a chiral carbon and has a pair of the enantiomer. One of the enantiomers (S) is the only active pain-killer, whereas other enantiomers (R) does not act as a pain killer at all. Thus to distinguish the stereoisomers we need to understand what are their absolute configurations.
The priority order:
i) Higher the atomic number higher the priority
ii) If two or more of the atoms that are bonded directly to the chiral center are the same, then prioritize these groups based on the next set of atoms
iii) If two atoms have substituents of the same priority, a higher priority is assigned to the atom with more of these substituents
iv) Atoms participating in double/triple bonds are considered to be bonded to an equivalent number of similar atoms by single bonds.
Now let’s see how to assign absolute configuration:
1) Prioritize and give numbers to each atom/group of atoms attached to the chiral carbon.
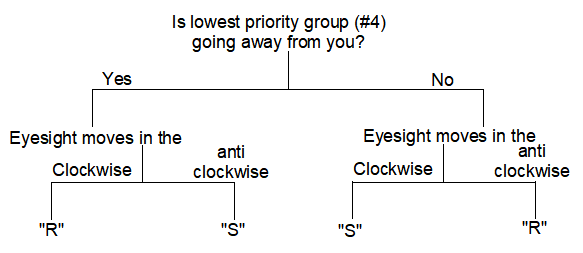
2) Move the eyesight as per the order from 1 to 3 (do not move till number 4!!).
3) Check if the lowest priority group (#4) if is going away from you or coming towards you.
3) If the lowest priority group (#4) is going away from you and the eyesight is moving in a clockwise direction, then the configuration is “R”.
If the eyesight moves in an anti-clockwise direction, then the configuration is “S”.
4) Further, if the lowest priority group is not going away from you and the eyesight moves in a clockwise direction, then the configuration is “S”.
Whereas, if the eyesight moves in an anti-clockwise direction, then the configuration is “R”.
Confused?? just follow the flow-chart:
Let’s deal with the real-life example:
why not consider: Ibuprofen?
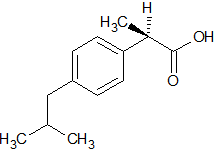
Priority order at chiral carbon:
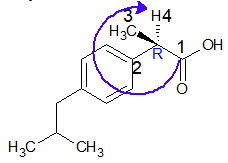
Lowest priority group, “H” is going away from the viewer.
and eyesight moves in clockwise direction, hence configuration is “R”.
This is inactive ibuprofen, don’t swallow the pill to treat your back pain.
🙂
Take another example of a little bit more complex molecule: (-)-Menthol, the main chemical that gives a refreshing flavor to the mint used in your ice mojito.
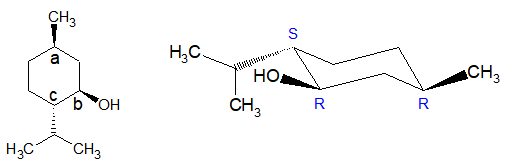
At chiral carbon Ca:
The priority order is: CH2-CH(OH) > CH2 > CH3 > H
The lowest priority group (here it is H) is going away from the viewer and eyesight moves in a clockwise direction. Hence configuration is “R”. 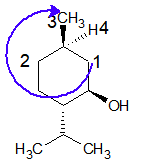
At chiral carbon Cb:
The priority order is: OH > CH(isoPr) > CH2 > H
The lowest priority group (here it is H) is going away from the viewer and eyesight moves in a clockwise direction. Hence configuration is “R”.
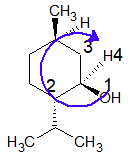
At chiral carbon Cc:
Priority order is: CH(OH) > isoPr > CH2 > H
The lowest priority group (here it is H) is coming towards the viewer and eyesight moves in a clockwise direction. Hence configuration is “S”.
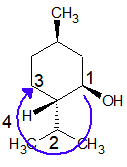
I understand in some molecules it may be tricky to get this configuration assigned, however, do let us know if you are unable to understand and want us to solve your queries. Your problems are not the end of the world, we are here to help you.

