Here we will see how to give IUPAC names to the organic molecules
How to name Alkanes using IUPAC guidelines:
- Find and name the longest continuous carbon chain.
- Identify and name groups attached to this chain.
- Number the chain consecutively, starting at the end nearest a substituent group.
- Designate the location of each substituent group by an appropriate number and name.
- Assemble the name, listing groups in alphabetical order using the full name (e.g. cyclopropyl before isobutyl). The prefixes di, tri, tetra, etc., used to designate several groups of the same kind, are not considered when alphabetizing.
- If there are two or more longest chains of equal length, the one having the largest number of substituents is chosen.
- If both ends of the root chain have equidistant substituents:
(i) begin numbering at the end nearest a third substituent, if one is present.
(ii) begin numbering at the end nearest the first cited group (alphabetical order).
Let’s look at some examples below: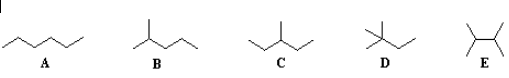 A: The name of the longest continuous carbon chain is hexane. There are no groups attached to this chain hence no substitution. Numbering may be done from either side. So the name is hexane.
A: The name of the longest continuous carbon chain is hexane. There are no groups attached to this chain hence no substitution. Numbering may be done from either side. So the name is hexane.
B: The name of the longest continuous carbon chain is hexane. There is a methyl group as a substituent for this chain. Numbering should be done in such a way that the methyl bearing carbon should get the least possible number.
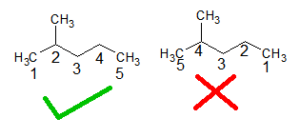
The location of the methyl group is, 2-methyl. There is only one substituent hence no alphabetical ordering needed. Thus the name is 2-methyl pentane.
Names of C, D, and E are 3-methyl pentane, 2,2-dimethyl butane, 2,3-dimethyl butane respectively.
Other examples:
CH3(CH2)2CH(CH3)CH2CH3
When viewing a condensed formula of this kind, one must recognize that parentheses are used both to identify repeating units, such as the two methylene groups on the left side, and substituents, such as the methyl group on the right side. This formula is elaborated and named as follows:
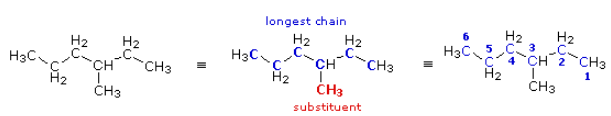
(CH3)2C(C2H5)2
The condensed formula is expanded on the left. By inspection, the longest chain is seen to consist of six carbons, so the root name of this compound will be hexane. A single methyl substituent (colored red) is present, so this compound is methyl hexane. The location of the methyl group must be specified since there are two possible isomers of this kind. Note that if the methyl group was located at the end of the chain, the longest chain would have seven carbons and the root name would be heptane, not hexane. To locate the substituent the hexane chain must be numbered consecutively, starting from the end nearest a substituent. In this case it is the right end of the chain, and the methyl group is located on carbon #3. The IUPAC name is thus: 3-methyl hexane.
(CH3)2C(C2H5)2
Again, the condensed formula is expanded on the left, the longest chain is identified (five carbons) and substituents are located and named. Because of the symmetrical substitution pattern, it does not matter at which end of the chain the numbering begins.
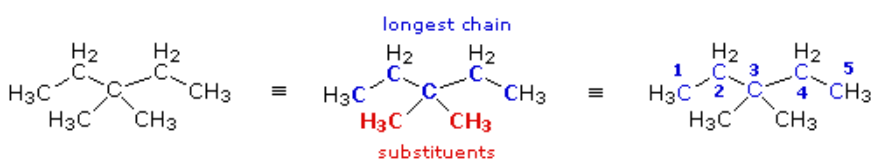
When two or more identical substituents are present in a molecule, a numerical prefix (di, tri, tetra, etc.) is used to designate their number. However, each substituent must be given an identifying location number. Thus, the above compound is correctly named: 3,3-dimethylpentane.
Note that the isomer (CH3)2CHCH2CH(CH3)2 would be named 2,4-dimethylpentane.
(CH3)2CHCH2CH(C2H5)C(CH3)3
This example illustrates some sub-rules of the IUPAC system that must be used in complex cases. The expanded and line formulas are shown below.
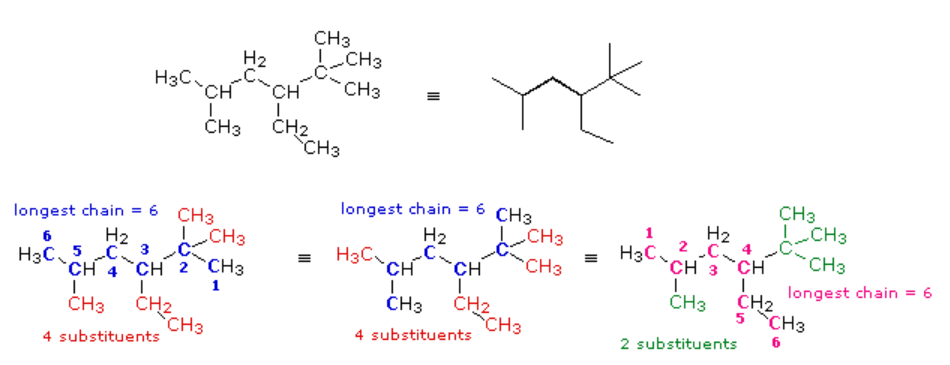
In this case, several six-carbon chains can be identified. Some (colored blue) are identical in that they have the same number, kind, and location of substituents. The IUPAC name derived from these chains will not change. Some (colored magenta) differ in the number, kind and location of substituents, and will result in a different name. From rule 1 above the blue chain is chosen, and it will be numbered from the right-hand end by application of the rule (i). Remembering the alphabetical priority, we assign the following IUPAC name: 3-ethyl-2,2,5-trimethylhexane.
The following are additional examples of more complex structures and their names.
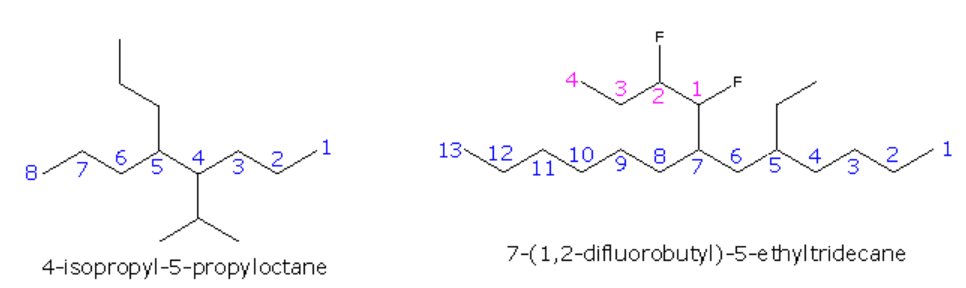
The formula on the right shows how a complex substituent may be given a supplementary numbering. In such cases the full substituent name is displayed within parenthesis and is alphabetized including numerical prefixes such as ‘di’.
IUPAC Rules for Cycloalkane Nomenclature
- For a monosubstituted cycloalkane, the ring supplies the root name (table above) and the substituent group is named as usual. A location number is unnecessary.
- If the alkyl substituent is large and/or complex, the ring may be named as a substituent group on an alkane.
- If two different substituents are present on the ring, they are listed in alphabetical order, and the first cited substituent is assigned to carbon #1. The numbering of ring carbons then continues in a direction (clockwise or counter-clockwise) that affords the second substituent the lower possible location number.
- If several substituents are present on the ring, they are listed in alphabetical order. Location numbers are assigned to the substituents so that one of them is at carbon #1 and the other locations have the lowest possible numbers, counting in either a clockwise or counter-clockwise direction.
- The name is assembled, listing groups in alphabetical order and giving each group (if there are two or more) a location number. The prefixes di, tri, tetra, etc., used to designate several groups of the same kind, are not considered when alphabetizing.
The following two cases provide examples of monosubstituted cycloalkanes.
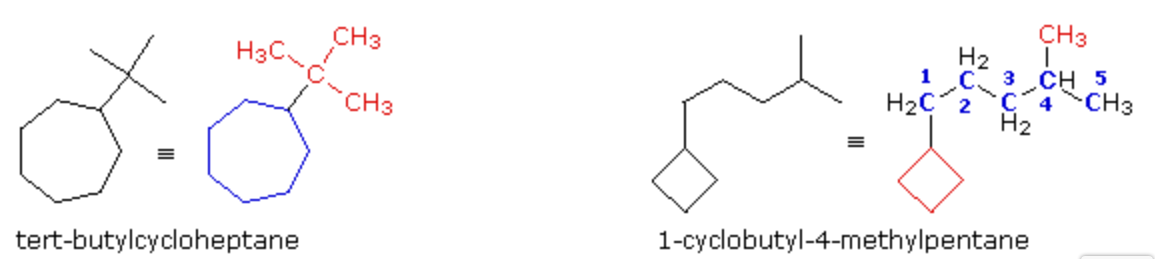
In the first case, on the left, we see a seven-carbon ring bearing a C4H9 substituent group. Earlier this substituent was identified as the tert-butyl group, so a name based on the cycloheptane root is easily written. In the second case, on the right, a four-carbon ring is attached to a branched six-carbon alkyl group. This C6H13 group could be named “isohexyl”, but a better approach is to name this compound as disubstituted pentane. The four-membered ring substituent is called a cyclobutyl group.
More highly substituted cycloalkanes are named in a similar fashion, but care must be taken in numbering the ring.
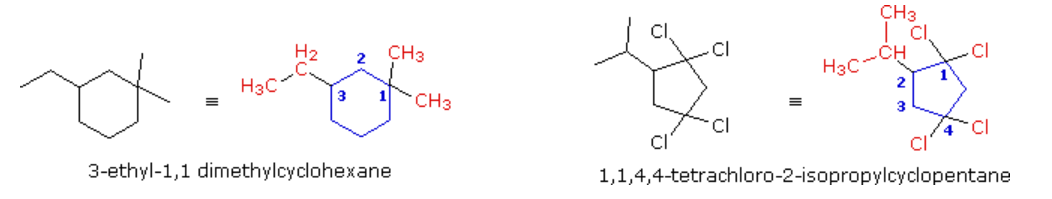
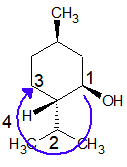
In the example on the left, there are three substituents on the six-membered ring and two are on the same carbon. The disubstituted carbon becomes #1 because the total locator numbers are thereby kept to a minimum. The ethyl substituent is then located on carbon #3 (counter-clockwise numbering), not #5 (clockwise numbering). Alphabetical listing of the substituents then leads to the name “3-ethyl-1,1-dimethylcyclohexane”, being careful to assign a locator number to each substituent. Note that if only one methyl substituent was present, the alphabetical citation rule would assign the ethyl group to carbon #1 and the methyl to #3. The second example, on the left, has five substituents, and the numbering is assigned so that the first, second and third arbitrarily chosen substituents have the lowest possible numbers (1,1 & 2 in this case).
Small rings, such as three and four-membered rings, have significant angle strain resulting from the distortion of the sp³ carbon bond angles from the ideal 109.5º to 60º and 90º respectively. This angle strain often enhances the chemical reactivity of such compounds, leading to ring cleavage products. It is also important to recognize that, with the exception of cyclopropane, cycloalkyl rings are not planar (flat).
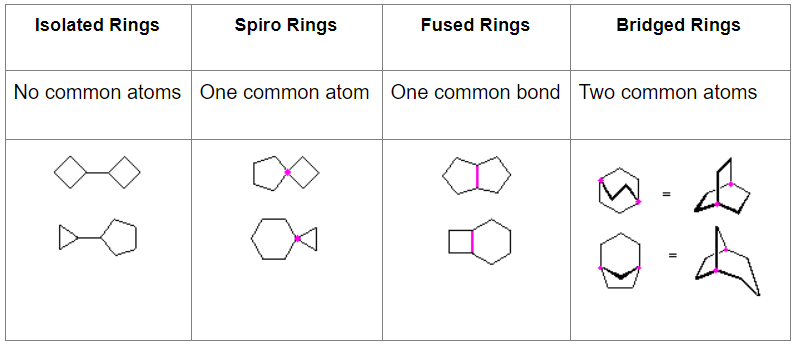
Hydrocarbons having more than one ring are common and are referred to as bicyclic (two rings), tricyclic (three rings) and in general, polycyclic compounds. The molecular formulas of such compounds have H/C ratios that decrease with the number of rings. In general, for a hydrocarbon composed of n carbon atoms associated with m rings the formula is: CnH(2n + 2 – 2m). The structural relationship of rings in a polycyclic compound can vary. They may be separate and independent, or they may share one or two common atoms. Some examples of these possible arrangements are shown in the following table.
Examples of Isomeric C8H14 Bicycloalkanes
Alkenes and Alkynes
IUPAC Rules for Alkene and Cycloalkene Nomenclature
- The ene suffix (ending) indicates an alkene or cycloalkene.
- The longest chain chosen for the root name must include both carbon atoms of the double bond.
- The root chain must be numbered from the end nearest a double bond carbon atom. If the double bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts.
- The smaller of the two numbers designating the carbon atoms of the double bond is used as the double bond locator. If more than one double bond is present the compound is named as a diene, triene or equivalent prefix indicating the number of double bonds, and each double bond is assigned a locator number.
- In cycloalkenes, the double bond carbons are assigned ring locations #1 and #2. Which of the two is #1 may be determined by the nearest substituent rule
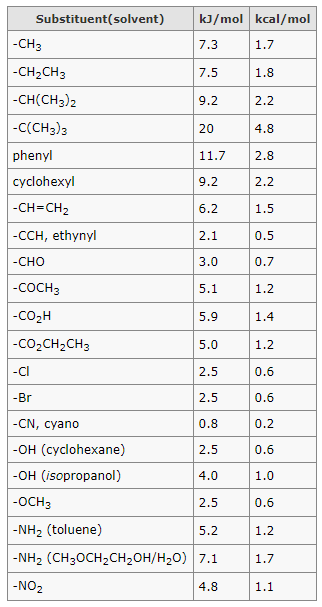
- Substituent groups containing double bonds are:
H2C=CH– Vinyl group
H2C=CH–CH2– Allyl group
IUPAC Rules for Alkyne Nomenclature
- The yne suffix (ending) indicates an alkyne or cycloalkyne.
- The longest chain chosen for the root name must include both carbon atoms of the triple bond.
- The root chain must be numbered from the end nearest a triple bond carbon atom. If the triple bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts.
- The smaller of the two numbers designating the carbon atoms of the triple bond is used as the triple bond locator.
- If several multiple bonds are present, each must be assigned a locator number. Double bonds precede triple bonds in the IUPAC name, but the chain is numbered from the end nearest a multiple bond, regardless of its nature.
- Because the triple bond is linear, it can only be accommodated in rings larger than ten carbons. In simple cycloalkynes the triple bond carbons are assigned ring locations #1 and #2. Which of the two is #1 may be determined by the nearest substituent rule.
- Substituent groups containing triple bonds are:
HC≡C– Ethynyl group
HC≡C–CH2– Propargyl group
- The root chain is that which contains the maximum number of multiple bonds.
- If more than one such chain is found, the longest is chosen as the root.
- If the chains have equal length the one with the most double bonds is chosen.
E.g.:
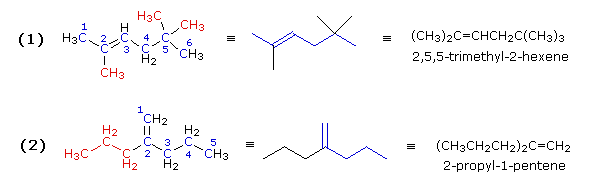
Both these compounds have double bonds, making them alkenes. In example (1) the longest chain consists of six carbons, so the root name of this compound will be hexene. Three methyl substituents (colored red) are present. Numbering the six-carbon chain begins at the end nearest the double bond (the left end), so the methyl groups are located on carbons 2 & 5. The IUPAC name is therefore: 2,5,5-trimethyl-2-hexene.
In example (2) the longest chain incorporating both carbon atoms of the double bond has a length of five. There is a seven-carbon chain, but it contains only one of the double bond carbon atoms. Consequently, the root name of this compound will be pentene. There is a propyl substituent on the inside double bond carbon atom (#2), so the IUPAC name is: 2-propyl-1-pentene.
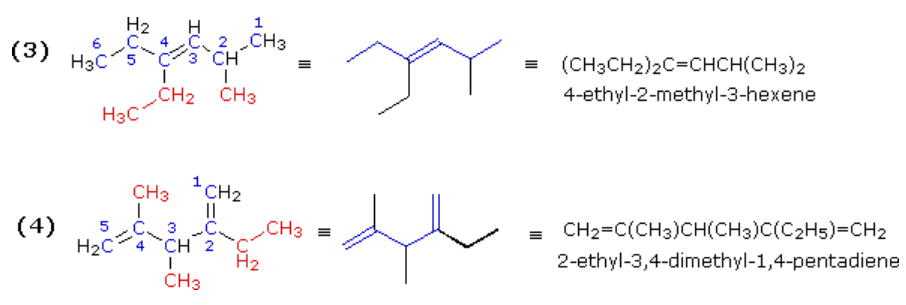
The double bond in example (3) is located in the center of a six-carbon chain. The double bond would therefore have a locator number of 3 regardless of the end chosen to begin numbering. The right hand end is selected because it gives the lowest first-substituent number (2 for the methyl as compared with 3 for the ethyl if numbering were started from the left). The IUPAC name is assigned as shown.
Example (4) is a diene (two double bonds). Both double bonds must be contained in the longest chain, which is therefore five- rather than six-carbons in length. The second and fourth carbons of this 1,4-pentadiene are both substituted, so the numbering begins at the end nearest the alphabetically first-cited substituent (the ethyl group).
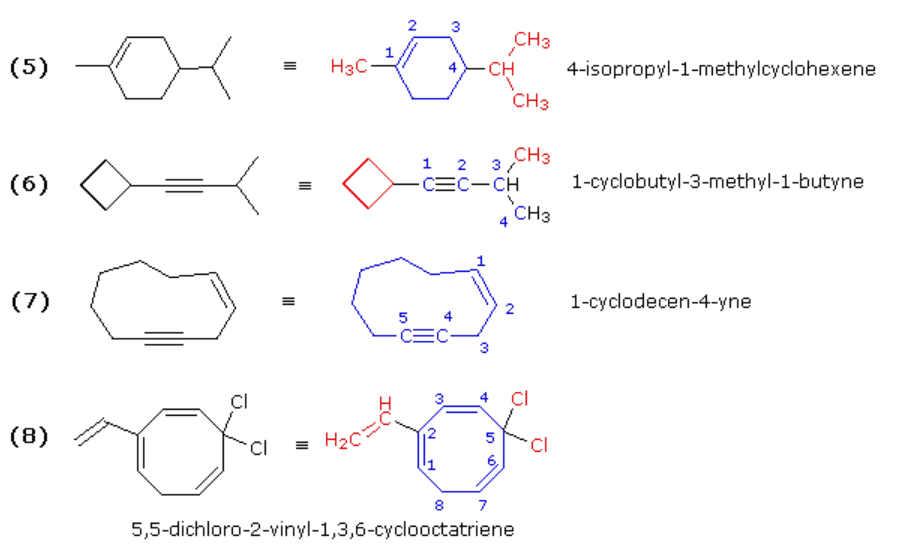
These examples include rings of carbon atoms as well as some carbon-carbon triple bonds. Example (6) is best named as an alkyne bearing a cyclobutyl substituent. Example (7) is simply a ten-membered ring containing both a double and a triple bond. The double bond is cited first in the IUPAC name, so numbering begins with those two carbons in the direction that gives the triple bond carbons the lowest locator numbers. Because of the linear geometry of a triple bond, a-ten membered ring is the smallest ring in which this functional group is easily accommodated. Example (8) is a cyclooctatriene (three double bonds in an eight-membered ring). The numbering must begin with one of the end carbons of the conjugated diene moiety (adjacent double bonds), because in this way the double bond carbon atoms are assigned the smallest possible locator numbers (1, 2, 3, 4, 6 & 7). Of the two ways in which this can be done, we choose the one that gives the vinyl substituent the lower number.
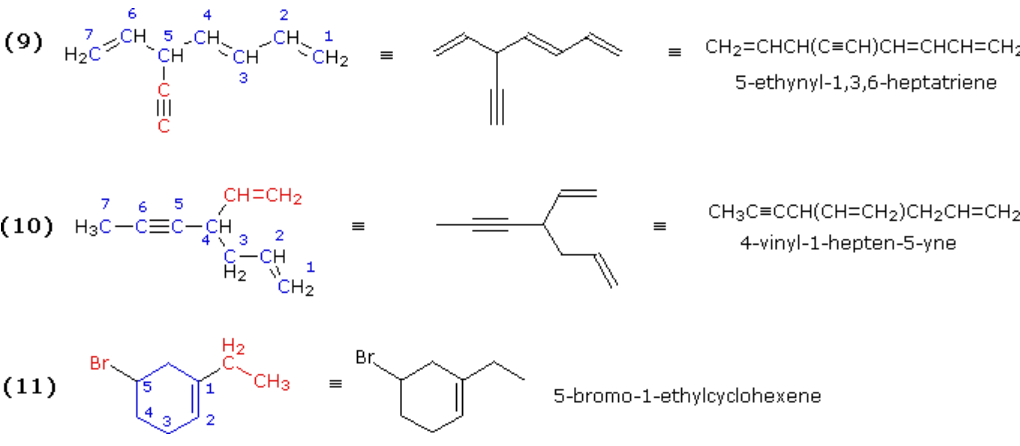
The first two acyclic cases are branched chains containing several multiple bonds. Example (9) has two possible seven-carbon chains, each having three multiple bonds. The one selected has three double bonds and the triple bond becomes a substituent group. In example (10) we find a six-carbon chain containing two double bonds, and a seven-carbon chain with a double and a triple bond. The latter becomes the root chain and the second double bond is a vinyl substituent on that chain. The last example (11) shows that in numbering a cycloalkene one must first consider substituents on the double bond in assigning sites #1 and #2. Here the double bond carbon atom to which the ethyl group is attached becomes #1 and the other carbon of the double bond is necessarily carbon #2. Sometimes this results in other substituents having high locator numbers, as does bromine in this case.
Benzene Derivatives
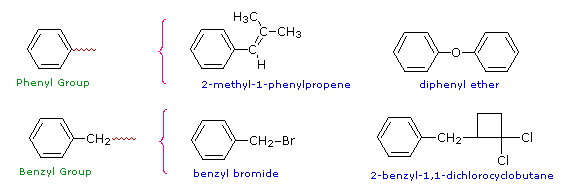
Two commonly encountered substituent groups that incorporate a benzene ring are phenyl, abbreviated Ph-, and benzyl, abbreviated Bn-. These are shown here with examples of their use. Be careful not to confuse a phenyl (pronounced fenyl) group with the compound phenol (pronounced feenol). A general and useful generic notation that complements the use of R- for an alkyl group is Ar- for an aryl group (any aromatic ring).
When more than one substituent is present on a benzene ring, the relative locations of the substituents must be designated by numbering the ring carbons or by some other notation. In the case of disubstituted benzenes, the prefixes ortho, meta & para are commonly used to indicate a 1,2- or 1,3- or 1,4- relationship respectively. In the following examples, the first row of compounds shows this usage in red. Some disubstituted toluenes have singular names (e.g. xylene, cresol & toluidine) and their isomers are normally designated by the ortho, meta or para prefix. A few disubstituted benzenes have singular names given to specific isomers (e.g. salicylic acid & resorcinol). Finally, if there are three or more substituent groups, the ring is numbered in such a way as to assign the substituents the lowest possible numbers, as illustrated by the last row of examples. The substituents are listed alphabetically in the final name. If the substitution is symmetrical (third example from the left) the numbering corresponds to the alphabetical order.
Group Names and Suffixes for Some Common Functional Groups
| Functional Class |
General Formula |
Group Name |
Suffix |
|
| Alcoholates |
-O(-) |
oxido- |
-olate |
| Alcohols |
-O-H |
hydroxy- |
-ol |
| Ethers |
-OR |
(R)-oxy- |
— |
| Peroxides |
-O-OR |
(R)-peroxy- |
— |
| Thiols |
-SH |
sulfanyl-
mercapto- |
-thiol |
| Sulfides |
-SR |
(R)-sulfanyl- |
— |
| Amines |
-NH2 |
amino- |
-amine |
| Carboxylic acids |
-CO2H |
carboxy- |
-carboxylic acid |
|
|
— |
-oic acid |
| Carboxylate Salts |
-CO2(-) M(+) |
— |
(cation) …carboxylate |
|
|
— |
(cation) …oate |
| Acid Halides |
-CO-X |
halocarbonyl- |
-carbonyl halide |
|
|
— |
-oyl halide |
| Amides |
-CONH2 |
carbamoyl- |
-carboxamide |
|
|
— |
-amide |
Esters
(of carboxylic acids) |
-COOR |
(R)-oxycarbonyl-
— |
(R)…carboxylate
(R)…oate |
| Hydroperoxides |
-O-OH |
hydroperoxy- |
— |
| Aldehydes |
-CH=O |
formyl- |
-carbaldehyde |
|
|
— |
-al |
| Ketones |
C=O |
oxo- |
-one |
| Imines |
C=NR |
(R)-imino- |
-imine |
| Nitriles |
-C≡N |
cyano- |
-carbonitrile |
|
|
— |
-nitrile |
| Sulfonic acid |
-SO2H |
sulfo- |
-sulfonic acid |
|
Note that only one functional group suffix, other than “ene” and “yne”, may be used in a given name. The following table gives the priority order of suffix carrying groups in arriving at an IUPAC name. When a compound contains more than one kind of group in this list, the principal characteristic group is the one nearest the top. All other groups are then cited as prefixes.
©Copyright Dr. SBP (ID:sbp150293)