*All the textbooks of organic chemistry teach you what are the energetics and how and why they make equatorial conformation more stable than the axial conformation. However, here I will be discussing how to calculate % of each conformation at a given temperature. And that is what examiners expect you to know well, isn’t it?
The “A-value” is a handy tool to understand how much the substituent, cost to go to most stable equatorial conformation. In short, A-value is the energy difference between axial and equatorial conformations. Let’s say if A-value is 7.3 kJ/mol for the methyl group, then the equatorial methylcyclohexane is more stable by 7.3kJ/mol than the axial methylcyclohexane.
Below is the table of A values and many common substituents:
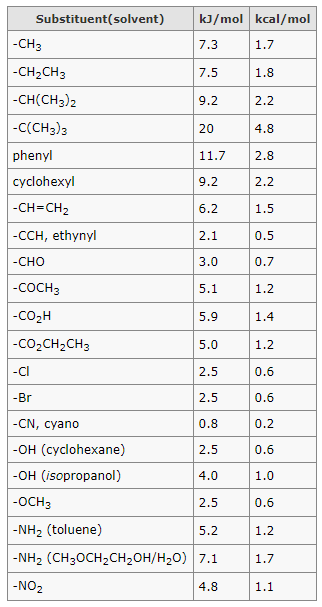
Reference: Stereochemistry of Organic Compounds, E. L. Eliel and S. H. Wilen.
Now, let’s do some calculations with different examples:
1) What is the percentage contribution of the axial and equatorial conformations of methylcyclohexane at 25, 50, 100 and 150°C?
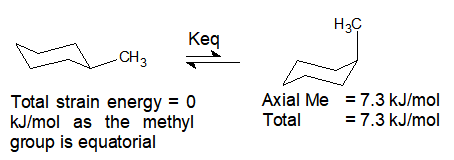

Thus at equilibrium and 25°C, 95% of equatorial-conformation and 5% of axial-conformation exists.
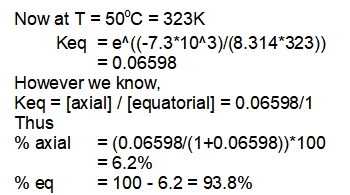
Thus at 50°C, 93.8% of equatorial conformation and 6.2% of axial conformation exists in equilibrium.
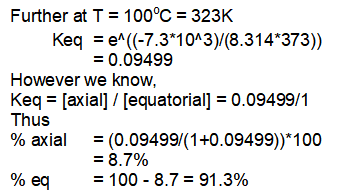
At 100°C, 91.3% of equatorial conformation and 8.7% of axial conformation exists in equilibrium.
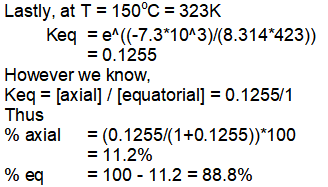
Thus we can say that at 150°C, 88.8% of equatorial conformation and 11.2% of axial conformation exists in equilibrium.
As we can see from the above calculations, as the temperature rises, the percentage contribution by equatorial conformation decreases, while that by axial conformation increases.
2) Calculate percent contribution by each conformation of cis-1-t.Butyl-2-methylcyclohexane at 25°C.
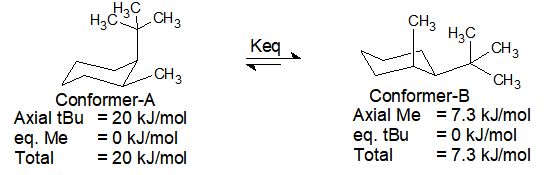
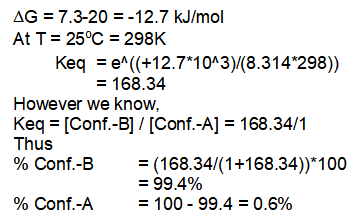
Thus it proves that bulky tert. butyl group is preferred over the equatorial position by more than 99% at 25°C.
3) Trans-3-bromocyclohexane-1-carbonitrile at 20°C.
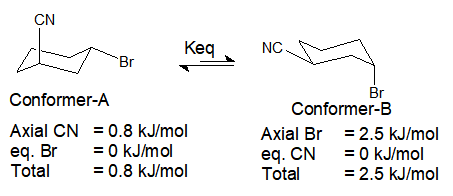
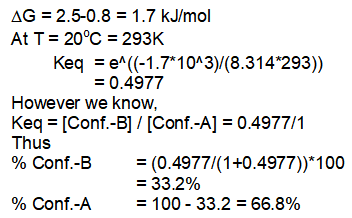
In this compound, conformation having Br equatorial is present in 66.8% and having Br at axial position is present in 33.2% at 20°C
I hope you will be able to just plug in your values and calculate any cyclohexane derivative conformation precentage at a given temperature.
If you have any question ask me any time on our homepage!
Attachment:
Below are some A-values, however you have to convert kcal/mol to kJ/mol using conversion factor:
1kcal/mol = 4.184kJ/mol
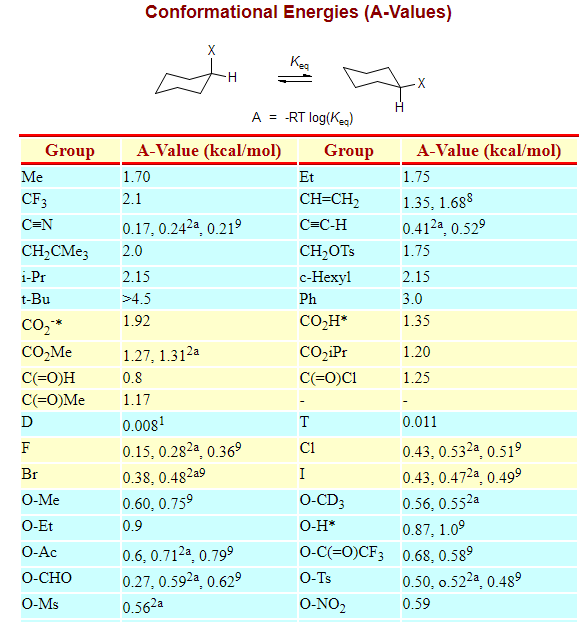
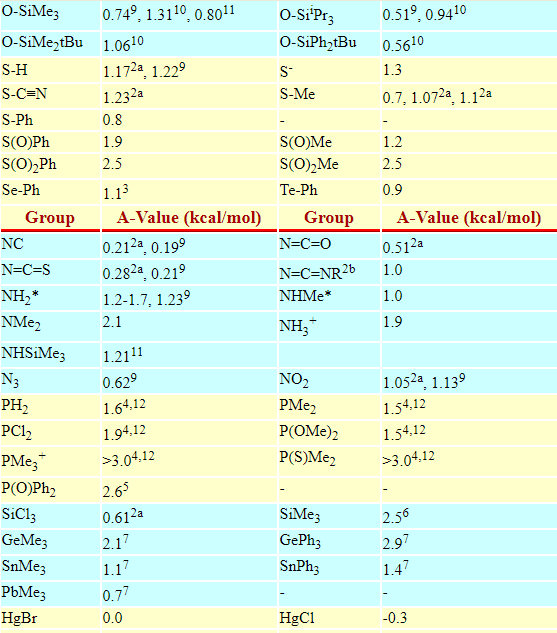
Please note that this particular blog-page is intended for Advance learners and those who know what it 1,3-diaxial interactions.
Dr. SBP (Online educator-OCT)

